Ammonium Chloride
Ammonium chloride is an inorganic compound that has consists of combination between the ammonium and chloride. It is naturally occurring in the mineralogic form. It appears as the white crystalline solid. some of the volcanic eruptions could be made by this chemical compounds. It added a flavouring agent like liquorice. The systematic IUPAC name is known as ammonium chloride. The chemical or molecular name of ammonium chloride is NH4Cl. It is also known as salt armoniack or salmiak.
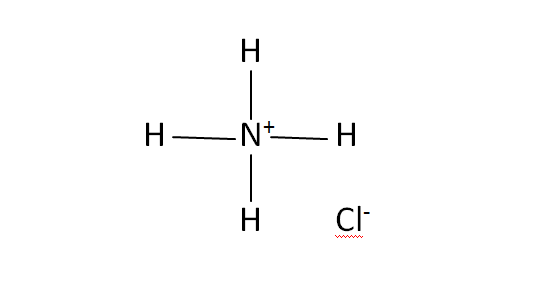
Structural Formula
This is the structural formula of the ammonium chloride:
Molecular Formula
The chemical formula of the ammonium chloride is NH4Cl. It has consists of four hydrogen and one chloride ions.
How the Ammonium Chloride is Prepared
By using the solvay process it can be prepared easily. The ammonia is reacted with the hydrogen chloride or hydrochloric acid to form the ammonium chloride. It is mainly used to manufacturing the fertilizers for the plant growth. The crystals are directly deposited at the gasesous state and it should be short lived and then easily dissolved in the water. The chemical reaction is given as follows.
NH3 + HCl → NH4Cl
Physical Properties
| Melting point | 338C |
| Boiling point | 520C |
| Molecular weight | 53.49g/mol |
| Density | 1.53g/cm3 |
| Solubility in water | 294g/L |
| Refractive index | 1.642 |
| Acidity | 9.24 |
| Magnetic susceptibility | -36.7×10-6cm3/mol |
| Appearance | White solid hygroscopic |
| Solubility | Solubility in methanol, ethanol and glycerol. |
| Vapour pressure | 6.5 kPa |
Chemical Properties
Ammonium chloride is a white solid hygroscopic. It is a strongly base chemical compounds. But it reacts with the both acid and basic forms. It has low density and high molecular weight. The vapour pressure is 6.5kPa. Ammonium chloride has low melting point and high boiling point. It has bitter in taste.
Uses
Ammonium chloride is used for fertilizer as nitrogen source. It is used lechlanche cells in aqueous solution. It is also used as a acidifier. It is used as glue for plywoods. It is used for cattles feed supplements. It is used for medicine as an expectorant.