Cadmium Nitrate
Cadmium nitrate is an inorganic chemical compound that has consists of cadmium and nitrate which has highly soluble in alcohols. That has been predominantly used in the field of agriculture for increasing the nitrogen minerals. It is a white crystalline hygroscopic solid. This is a major component for the explosive mixtures in quarrying, mining and civil constructions. The systematic IUPAC name is known as cadmium nitrate. The chemical or molecular name of cadmium nitrate is Cd(NO3)2. It is also known as nitric acid or cadmium salt.
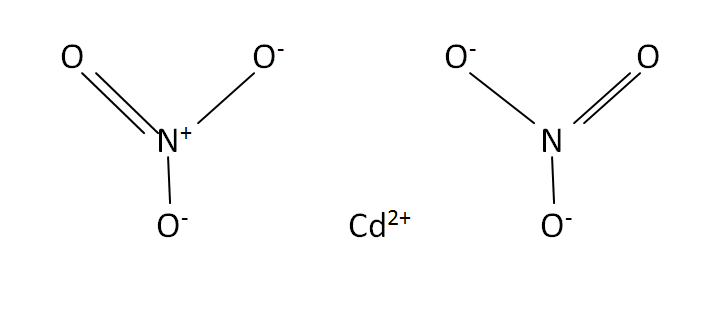
Structural Formula
This is the structural formula of the cadmium nitrate:
Molecular Formula
The chemical formula or molecular formula of the cadmium nitrate is Cd(NO3)2. It has consists of cadmium and two nitrate ions.
Preparation Method of Cadmium Nitrate
By using the crystallation process it can be prepared by the two methods. In the first method the calcium oxide is reacted with the nitric acid and it gives the cadmium nitrate is the product and water is the byproduct. In the second method it is reacted between the cadmium carbonate and nitric acid to give the cadmium nitrate as the product and carbon dioxide, water as the byproduct.
CdO + 2 HNO3 → Cd(NO3)2 + H2O
CdCO3 + 2 HNO3 → Cd(NO3)2 + CO2 + H2O
Physical Properties
| Melting point | 561C |
| Boiling point | 132C |
| Molecular weight | 164.088g/mol |
| Density | 2.5g/cm3 |
| Solubility in water | 109.7g/100mL |
| Refractive index | 1.569 |
| Crystal structure | Cubic |
| Magnetic susceptibility | -5.51×10-6cm3/mol |
| Appearance | White crystals hygroscopic |
| Solubility | Soluble in acids, ammonia and alcohols. |
| Odor | odourless |
Chemical Properties
Cadmium nitrate is corrosive material and reacts violently with the water. It is a non inflammable substances. It is related to organic chemistry. It is antifluorite in structure. It looks like a white solid in the appearance. The boiling point is high and the melting point is low. It has low molar mass and high density.
Uses
Cadmium nitrate is used in agricultural fields as a fertilizer. It is an oxidizing agent. It is used as a raw material making other nitrate compounds. It is used for light industry and also fireworks.