Ammonium Nitrite Formula
Ammonium nitrite is the inorganic compound that has consists of nitric and ammonia acid. It is naturally abundant in the form of air and it can be obtained through the absorption of equal parts of nitric oxide and nitrogen dioxide in liquid ammonia. Even at room temperature, it can be decomposed into the water and the nitrogen. The systematic IUPAC name is known as ammonium nitrite. The chemical or molecular formula of ammonium nitrite is NH4NO2.
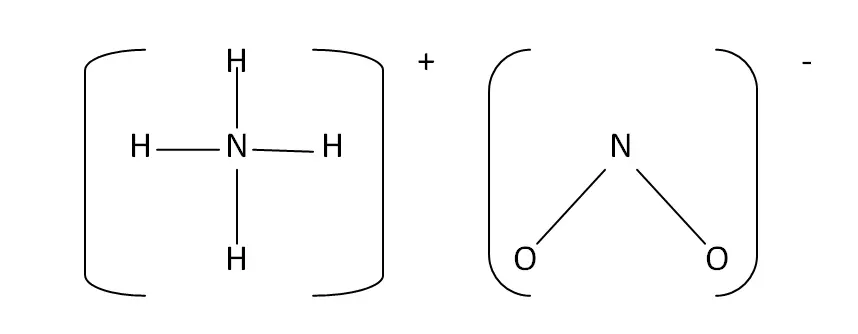
Structural Formula
This is the structural formula of the ammonium nitrite:
Chemical Formula
The chemical formula of the ammonium nitrite is NH4NO2.
Preparation Method
When the nitrogen is reacts with water in the form of air that yields ammonium nitrite. It can be produced by the heat we given to the nitrogen and water.
N2 + 2H2O → NH4NO2
In the precipation process, the nitrite or barium is reacted with the other chemical such as ammonium sulfate, potassium nitrate with ammonium perchlorate. Then it can be filtered which is soluble in water and colourless. It gives the result of ammonium nitrite.
In the another methor the silver nitrate is reacted with ammonium chloride then it gives or yields ammonium nitrite and silver chloride.
2AgNO2 + NH4Cl → NH4NO2 + 2AgCl
Physical Properties
| Melting point | Decomposes |
| Molecular weight | 64.06g/mol |
| Density | 1.69g/cm3 |
| Solubility in water | 118.3g/100ml |
| Appearance | Pale yellow crystals |
Chemical Properties
It can explode at a temperature between 60 to 70C and it could be decomposed fast in the aqueous solutions. If any difference occur in the ph level then it may lead to explosion within the seconds. Because it is higher stable in the ph and maintained at the low temperature. It is important to maintain the ph level when the ammonia is added to the solution. The ratio will be more than 10% for ammonium nitrite to ammonia.
Uses
The ammonium nitrite is commonly used for making the explosives at high quality. This is used for the production of ammonium cobalt nitrite. It is used for the agricultural pesticides to kill the insecticides. In the medical field, it is used to produce the micro biocide for killing the microbes. It is also used for manufacturing the nitrogen gas. It is used as well as rodenticides.