Ammonium Nitrate Formula
Ammonium nitrate is a chemical compound that has consists of ammonia and nitrate which has highly soluble in water. That has been predominantly used in the field of agriculture for increasing the nitrogen minerals. It is a white crystalline solid. This is a major component for the explosive mixtures in quarrying, mining and civil constructions. The systematic IUPAC name is known as ammonium nitrate. The chemical or molecular formula of ammonium nitrate is NH4NO3.
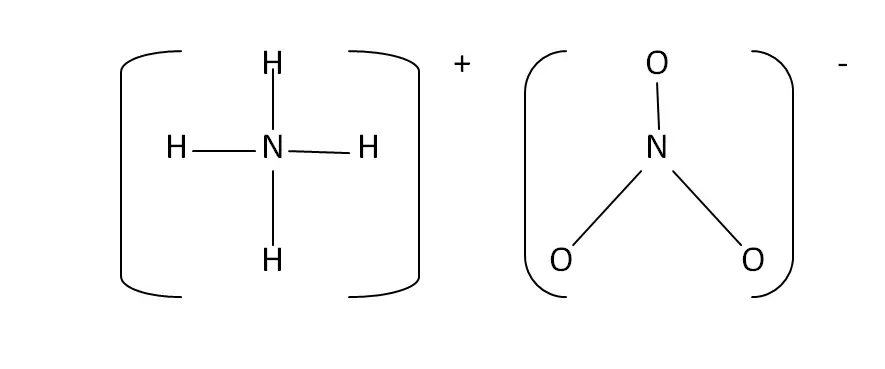
Structural Formula
This is the structural formula of the ammonium nitrate:
Chemical Formula
The chemical formula of the ammonium nitrate is NH4NO3.
Preparation Method
It is a acid base reactions. When the exothermic reactions is catalyst it is formed the 83% concentration of the ammonium with the excess of water. The ammonia is reacted with the nitric acid it gives the product of ammonium nitrate acid.
HNO3 + NH3 → NH4NO3
By using the habers process, the hydrogen and nitrogen is formed the ammonia. In the chemical reactions the both anion and cation take part during the reactions.
Physical Properties
| Melting point | 169.6C |
| Boiling point | 210C |
| Molecular weight | 80.043g/mol |
| Density | 1.72g/cm3 |
| Solubility in water | 1024g/100ml(100C) |
| Appearance | White crystalline solid |
| Crystal structure | trigonal |
Chemical Properties
It is pleasant in smell and odourless. These chemicals are decomposed at the temperature of 300C. It is more explosive and hazardous substances to the environment. The crystalline phases are changed to the dependent of the atmospheric pressure. When the temperature is more than upto 125.2 then it is called the cubic symmetry. If the temperature is below 16.8 then it will be the tetragonal symmetry.
Uses
It is mainly used for manufacturing the urea with the nutrient of nitrogen 34% added. It is used as the fuel for rockets propellants in the form of explosive substances. From the mining and quarrying areas it has the get the benefits of water resistance, high detonation velocity and stones for the construction of buildings. It does not any hazardous for the health.