Aluminium Hydroxide Formula
Aluminium hydroxide is a chemical compounds that consists of mineral gibbsite which is also known as hydrargilite. This aluminium hydroxide is an amphoteric which is closely related to the aluminium oxide hydroxide AlO(OH) and aluminium oxide (Al2O3). The aluminium ore bauxite contains these two major components together. The systematic IUPAC name is known as trihydroxidoaluminium. The chemical or molecular formula of aluminium hydroxide is Al(OH)3.
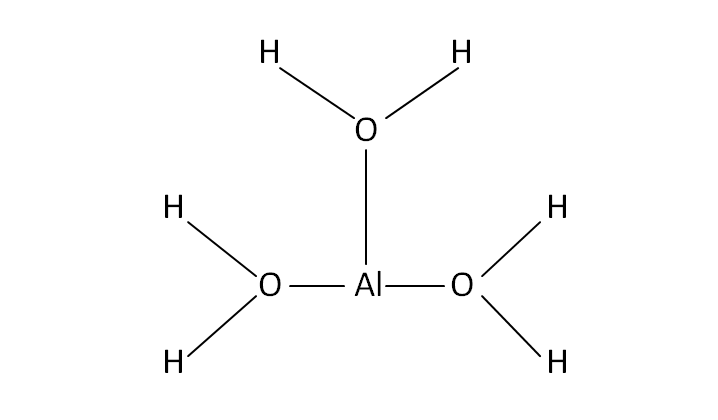
Structural Formula
This is the structural formula of the aluminium hydroxide:
Chemical Formula
The chemical formula of the aluminium hydroxide is Al(OH)3.
Preparation of aluminium hydroxide
By the bayer’s process, it is commercially manufactured in the dissolving bauxite in sodium hydroxide at the temperature 270C. then it is removed by the bauxite tailings the waste solid products and the aluminium hydroxide is precipated from the sodium aluminate. This is known as the calcinations process. It has both acid and base properties. In acidic it act as the bronsted lowry base. In the basic, it act as a lewis acid.
3HCl+Al(OH)3 → AlCl3+3H2O
Al(OH)3+OH– → Al(OH)4–
Physical Properties
| Melting point | 300C |
| Molecular weight | 78.00g/mol |
| Density | 2.42g/cm3 |
| Solubility in water | 0.0001g/100mL |
| Solubility product | 3×10-34 |
| Appearance | White amorphous powder |
| Acidity | >7 |
| Isoelectric point | 7.7 |
Chemical Properties
It is used for polymer applications to use as a fire retardant filler. It act as a similar to the good retardant properties. This can be filled throught the smoke suppressant and giving off water vapour. It has been easily structured the range of polymers such as in polysters, ethyl vinyl chloride, epoxies and polyvinyl chloride.
Uses
It is used in the manufacturing of calcined aluminas, aluminium nitrate, sodium aluminate, zeolites and polyaluminium chloride. It can be used to form the gels that has contains the flocculants with the water purification. It can be dehydrated by the water miscible and non aqueous solutions which is formed the amorphous aluminium hydroxide powder. This can be also used in the manufacturing of anti-oxidants. It can be treated as the ulcers and constipations.