Zinc Bromide
It is an inorganic compound that has consists of colourless with a high solubility in water. It has a transparent film against the radiations. Whenever it is soluble in organic solvents with good solubility. It could be processed slowly but exceptions level of radiation is high. The systematic IUPAC name is known as zinc bromide. The chemical or molecular formula of acetamide is ZnBr2.
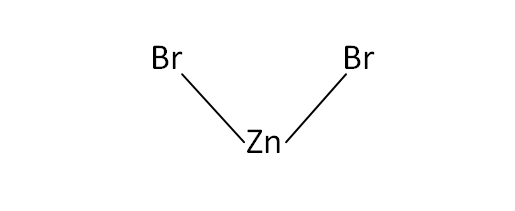
Structural Formula
This is the structural formula of the zinc bromide:
Chemical Formula
The chemical formula of the zinc bromide is ZnBr2.
Structure
It contains the super tetrahedral in the composition of linked their vertices to form a three dimensional structure. It could be the length of zn-br bond is upto 221pm.
Preparation Method
When the zinc oxide is reacted with the hydrobromic acid to form the ZnBr2.2H2O. then it is treated with the dehydration of the hot CO2 with the anhydrous material of zinc metal and bromine. It gives the sublimation of stream in the hydrogen bromide.
ZnO + 2 HBr + H2O → ZnBr2.2H2O
Zn + 2 HBr → ZnBr2 + H2
Physical Properties
| Melting point | 394C |
| Boiling point | 697C |
| Molecular weight | 255.18g/mol |
| Density | 4.20g/cm3 |
| Solubility in water | 675g/100mL |
| Refractive index | 1.5452 |
| Appearance | White crystalline powder |
| Solubility | Very soluble in alcohol |
Chemical Properties
Zinc bromide is a non-inflammable chemicals that is used to easily handle. By due to high acidity and osmolarity it causes the corrosion and other related problems. It is non hazardous to the environment. It is naturally white crystalline powder and hygroscopic structure. Zinc bromide is a colourless salt.
Uses
Zinc bromide is mainly used in oil industry, electrical industry and metal working companies. Zinc bromide is used as Lewis acid in organic chemistry. It is used in transparent film shield against radiation. It is widely used in electrical field. Zinc bromide is used in oil industry to displace mud. It is also used in metal working companies. It is the ionic compound used in various industrial field.