Aluminium Sulfide
Aluminium sulfide is a chemical compound that looks like a gray in the appearance. At the air it is formed the hydrogen sulfide. It possess a strong smelling agent. It has formed the six crystalline such as alpha, beta and gamma. In the themite like reaction the sulfur and aluminium powders can take place. It released the hydrogen sulfide when it is hydrolysed.The systematic IUPAC name is known as aluminum sulfide. The chemical or molecular formula of aluminium sulfide is Al2S3.
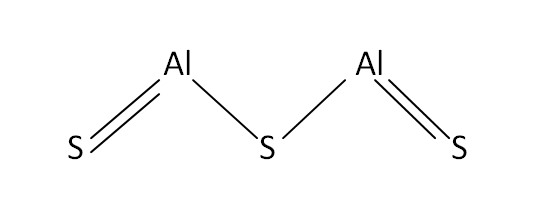
Structural Formula
This is the structural formula of the aluminium sulfide:
Chemical Formula
The chemical formula of the aluminium sulfide is Al2S3.
Preparation Method
In an exothermic process the ignition of the elements can take place for prepared the aluminium sulfide. The product is created at the fused form. It melts away from the steel at 1100C. it is highly stabilized form with the structure in the hexagonally closed pack arrangement of the sulfide anions.
2 Al + 3 S → Al2S3
Physical Properties
| Melting point | 1100C |
| Boiling point | 1500C |
| Molecular weight | 150.158g/mol |
| Density | 2.02g/cm3 |
| Solubility in water | Hydrolyses to release H2S |
| Solubility | Insoluble in acetone |
| Crystal structure | Trigonal |
| Appearance | Gray solid |
Chemical Properties
Aluminium sulfide is highly moisturizing in the atmosphere. The melting and boiling point is very high. It has low density and medium molecular weight. It is trigonal in structure. The solubility of the aluminium bromide is insoluble in the acetone. It looks like a gray solid in the appearance.
Uses
Aluminium sulfide is used for hydrogen sulphide. Aluminium sulfide is used in tanning and paper making industry. It is also used as a organic compounds such ethanethiol.