Aluminium Oxide
Aluminium oxide is a chemical compound that is the combination of aluminium and oxygen ions to make a perfect aluminium oxide. This is an ionic compound that generates the electrons pair with the oxygen form the alumina. So it occurs naturally in the form of polymorphic phase stage as the mineral corundum. It is significant to use the alumina metal to abrasive process in the industry. The systematic IUPAC name is known as aluminum oxide. The chemical or molecular formula of aluminium oxide is Al2O3.
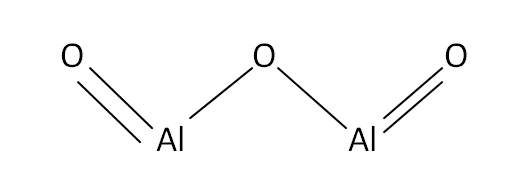
Structural Formula
This is the structural formula of the aluminium oxide:
Chemical Formula
The chemical formula of the aluminium oxide is Al2O3.
Preparation Method
A mixture of the aluminium hydroxide with the sodium hydroxide to prepare for the bauxites. It is the principal ore of aluminium. With the impurities of iron oxides, quartz and clay minerals. It is typically purified by the bayers process. Then further it is treated under the bayer liquor is cooled, it precipitates and leaving the solution. Finally it undergoes the treatment of calcinated over heating at 1100C and it gives the product of aluminium oxide.
Al(OH)3 + NaOH → NaAl(OH)4
2Al(OH)3 → Al2O3 + 3 H2
Physical Properties
| Melting point | 2072C |
| Boiling point | 2977C |
| Molecular weight | 101.96g/mol |
| Density | 3.95g/cm3 |
| Solubility in water | Insoluble |
| Solubility | Insoluble in all solvents |
| Crystal structure | Trigonal |
| Magnetic susceptibility | -37.0×10-6cm3/mol |
| Appearance | White solid |
| Odor | odourless |
Chemical Properties
The melting point and the boiling point is very high. It is trigonal in structure. It is insoluble in all the solvents. It has a low density and medium molecular weight. It is white solid in the appearance. It is odorless.
Uses
Aluminium oxide is used in formulation in glass. It is also used as a catalyst. It is widely used for water purification to remove water from gas streams. Aluminium oxide is used sodium vapour lamps. Usually it is used in sandpaper as an abrasive. It is used in cosmetics such as nail polish, lipsticks.