Sodium Dichromate
Sodium dichromate is an inorganic compound that has consists the chromium ores are processed and producing the salts based on the chromium materials. It is similar in the structure of potassium dichromate. It has lower in the equivalent weight. It is twenty times more than the potassium salt. The systematic IUPAC name is known as sodium dichromate . The chemical or molecular formula of sodium dichromate is Na2Cr2O7. It is also known as chromic acid disodium salt.
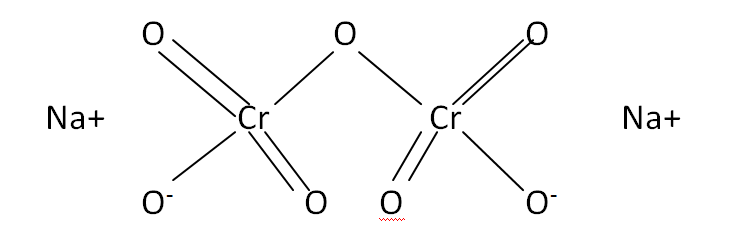
Structural Formula
This is the structural formula of the sodium dichromate:
Chemical Formula
The chemical formula of the sodium dichromate is Na2Cr2O7.
Preparation Method
In the presence of oxygen the sodium chromate is reacted with the carbon dioxide and water, it produces the sodium dichromate as the product and sodium carbonate as the byproduct at the temperature above 1000C. In the another way when the sulfuric acid is reacted with the sodium chromate it gives the formation of sodium dichromate and sodium sulfate and water is the byproduct. The chemical reaction is given as follows.
2Na2CrO4 + 2CO2 + H2O → Na2Cr2O7 + 2NaHCO3
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4+ H2O
Physical Properties
| Melting point | 356.7C |
| Boiling point | 400C |
| Molecular weight | 261.97g/mol |
| Density | 2.52g/cm3 |
| Solubility in water | 73g/100ml(25C) |
| Refractive index | 1.661 |
| Magnetic susceptibility | -14.1×10-6cm3/mol |
| Appearance | Bright orange |
| Solubility | Soluble in methanol and ethanol. |
Chemical Properties
Sodium dichromate is highly inflammable substances. The main hazards are irritant to the eyes and highly corrosive resistant. It is slightly soluble in methanol and ethanol. It is cubic in the structure . It looks like a bright orange in the appearance. The boiling point is high and the melting point is low. It has low density and high molar mass.
Uses
Sodium dichromate is used to manufacturing the dyes for preventing the corrosive inhihitor in the metals. In the laboratory it is producing the organic chemicals. These chemicals is help to wood preservative from the termites. It is used to drill the muds.