Ammonium Bromide
Ammonium bromide is a chemical compound that has the odourless white granules and has a saline taste. It is derived from the hydrobromic acid. It is easily soluble in water. It converts the bromide to bromine when it exposure gradually on the air. The systematic IUPAC name is known as ammonium bromide. The chemical or molecular formula of ammonium bromide is NH4Br.
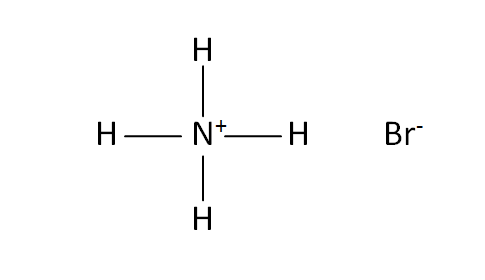
Structural Formula
This is the structural formula of the ammonium bromide:
Chemical Formula
The chemical formula of the ammonium bromide is NH4Br.
Preparation Method
The ammonium bromide is prepared by the ammonia is reacting with the hydrogen bromide. The chemical reaction is as follows.
NH3 + HBr → NH4Br
The another method to produce the ammonium bromide is ammonia reacting with the iron bromide which is passing on the aqueous solution.
2NH3 + FeBr2 + 2H2O 2NH4Br + Fe(OH)2
Physical Properties
| Melting point | 235C |
| Boiling point | 452C |
| Molecular weight | 97.943g/mol |
| Density | 2.429g/cm3 |
| Solubility in water | 60.6g/100mL |
| Refractive index | 1.712 |
| Crystal structure | Isometric |
| Magnetic susceptibility | -47.0×10-6cm3/mol |
| Appearance | White powder hygroscopic |
Chemical Properties
The melting and boiling point of the ammonium bromide is balanced at room temperatures. The magnetic susceptibility has been very low. It is isometric in structure. It is insoluble in alcohols. It has a low density and low molecular weight. It is less hazardous to the environment.
Uses
Ammonium bromide is used for photography . It’s also used for waterproofing wood. Ammonium bromide is used as a corrosive inhibitor. It used for photography for clearance of photo.