Zinc Chloride
Zinc chloride is a chemical compound that has consists of zinc and chlorine. Zinc chloride is an ionic salt and it possessed the minerals which is essential for synthesis of proteins, cholesterol, fats and lipids. This is a colorless liquid having the hygroscopic qualities. The water molecules are absorbed by the zinc chloride which is present in the environment. Zinc chloride has corrosive properties towards the metals. It affects or irritates the skin, eyes and mucous membrances. The systematic IUPAC name is known as zinc chloride. The chemical or molecular formula of acetamide is ZnCl2.
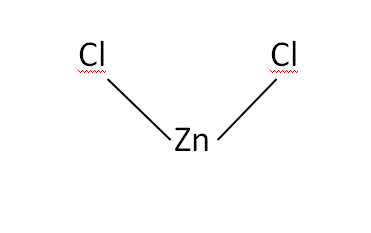
Structural Formula
This is the structural formula of the zinc chloride:
Chemical Formula
The chemical formula of the zinc chloride is ZnCl2.
Preparation Method
When the hydrogen chloride is reacted with the zinc it gives a result the formation of zinc chloride. The chemical reaction is as follows.
Zn + HCl → ZnCl2 + H2
In another way it is produced by the hydrochloric acid is reacted with the zinc sulphide over heated at some temperature then it gives the formation of zinc chloride. The chemical reaction is given below.
ZnS + 2HCl → ZnCl2 + H2S
Physical Properties
| Melting point | 275C |
| Boiling point | 756C |
| Molecular weight | 136.286g/mol |
| Density | 2.91g/cm3 |
| Solubility in water | Soluble |
| Odor | Odorless |
| Crystal structure | Tetrahedral |
| Magnetic susceptibility | -65.0×10-6cm3/mol |
| Appearance | Colourless or white crystals |
Chemical properties
Zinc chloride is deliquescent in nature and soluble in water, glycerol, ether and alcohol. The solution becomes acidic when the zinc chloride is dissolved in the water. the pH of this solution is having a concentration of aqueous solution is 6M that is equal to 1.
Uses
Zinc chloride is used in dry cells as an electrolyte. It is widely used in condensing agent, dehydrating agent, deodarent and disinfectant. In room temperature zinc chloride in solid and it has white appearance and odourless. It is highly viscous and low electrical conductivity. Zinc chloride is soluble in acetone, ethanol and glycerol. It is used in fingerprint detection. Zinc chloride is useful to preparation of alkyl chloride. This compound is acts as catalyst aromatization reaction in molten state.