Strontium Chloride
Strontium chloride is an inorganic salt that has consists the strontium and chloride. In the aqueous solution it forming the compounds of strontium and easily converted to the typical salt. It also acts as the intermediate compound between the barium chloride and calcium chloride. The polarization of the electron core may causes the distortion and it could be directly interacts with the strontium-chloride bonds. The systematic IUPAC name is known as strontium chloride. The chemical or molecular formula of strontium chloride is SrCl2. It is also known as strontium II chloride.
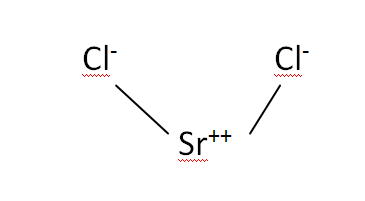
Structural Formula
This is the structural formula of the strontium chloride:
Chemical Formula
The chemical formula of the strontium chloride is SrCl2.
What Method the Strontium Chloride Prepared
The strontium chloride is prepared by treating the chemical compounds in the aquous solutions. When the strontium hydroxide or strontium carbonate is reacted with the hydrochloric acid, it will give the product as strontium chloride and water is given as the byproduct. Then it gives the hexahydrate when this is undergoes to the crystallization. The chemical reaction is given as follows.
Sr(OH)2 + 2 HCl → SrCl2 + 2 H2O
Physical Properties
| Melting point | 874C |
| Boiling point | 1250C |
| Molecular weight | 158.53g/mol |
| Density | 3.052g/cm3 |
| Solubility in water | 53.8g/100mL(20C) |
| Refractive index | 1.650 |
| Crystal structure | Deformed rutile structure |
| Magnetic susceptibility | -63×10-6cm3/mol |
| Appearance | White crystalline solid |
| Solubility | Slightly soluble in ethanol and acetone. Insoluble in ammonia. |
Chemical Properties
Strontium chloride is caused the irritant to the eyes and skin. It appears as a white crystalline solid. It has deformed rutile in the structure. It is insoluble in the ammonia. The boiling point is greater than the melting point.
Applications
Strontium chloride is used for red colouring agent in fireworks. It is widely used for the treatment of bone cancer. It can be reduced the tooth sensitivity. It acts as the corrosive inhibitor for aluminium metals.