Sodium Acetate Formula
Sodium acetate is one of the chemical compound than comprises one sodium atom, two carbon atoms, two oxygen atoms and three hydrogen atoms. It is occurred in the sodium salt when it is heated with high boiling point. It can be easily soluble in water and hygroscopic in nature. When it is heated it smells look like a vinegar or acetic acid. This compound has been odourless. The systematic IUPAC name is known as sodium acetate. The chemical or molecular formula of sodium acetate is CH3COONa.
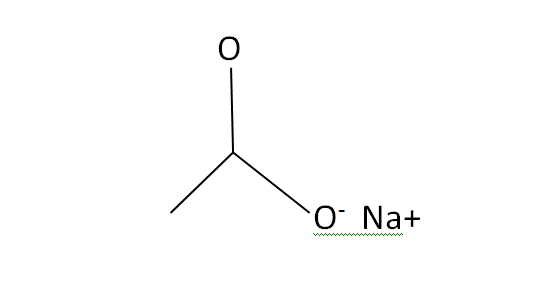
Structural Formula
This is the structural formula of the sodium acetate:
Chemical Formula
The chemical formula of the sodium acetate is CH3COONa.
Preparation Method
It can be prepared with the help of baking soda and vinegar. You can take the one spoonful of baking soda and add vinegar while stirring the mixture. When the bubbles is stoping then you can also stop to add the vinegar. Because the sodium bicarbonate is converted into the sodium acetate and carbon dioxide. At the supersaturated point, it will absorb all the water while cooling at the room temperature. Then scrap the gel until the form of sodium acetate powder.
When the acetic acid is reacted with the baking soda, the sodium acetate is prepared and carbonic acid is the byproduct.
CH3COOH+NaHCO3 → CH3COONa+H2C03
Physical Properties
| Melting point | 324C |
| Boiling point | 881.4C |
| Molecular weight | 82.034g/mol |
| Density | 1.528g/cm3 |
| Solubility in water | 162.9g/100mL(100C) |
| Refractive index | 1.464 |
| Crystal structure | Monoclinic |
| Magnetic susceptibility | -37.6×10-6cm3/mol |
| Appearance | White deliquescent powder |
Chemical Properties
It is naturally hazardous for health. You can handle this safely otherwise it occurs eye irritation, skin rashes and problems arises in the respiratory system. When it is heated above 58C it could be loses the hydration capacity and the exothermic process is occurred.
Uses
Sodium acetate is used in dialysis in the source of ion. It is widely used in textile industry for dye aniline. Sodium acetate is acts as a concrete sealent. Usually used in hot ice and hand warmers. It is used to rid up of the static electricity. Pickling agent is used in chrome tanning. Sodium acetate used in textiles to dye our sarees,shirts etc. Chrome tanning technique is very useful to sodium acetate for pickling.