Potassium Permanganate
Potassium permanganate is an inorganic compound and has a strong oxidizing agent. The potassium has a positive charge(cation) and the permanganate is a negative charge(anions). This chemical compound is dissolves in water. yearly it was produced the 20 to 30 thousand tonnes from the worldwide. The systematic IUPAC name is known as potassium permanganate . The chemical or molecular formula of potassium permanganate is KMnO4. It is also known as the hypermangan or condys crystals.
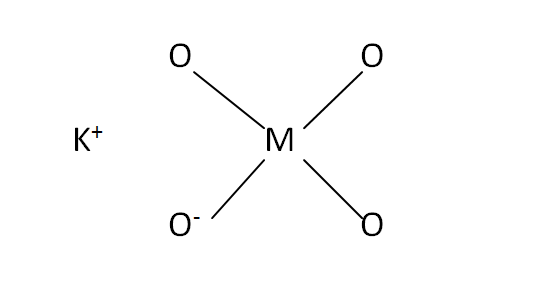
Structural Formula
This is the structural formula of the potassium permanganate:
Chemical Formula
The chemical formula of the Potassium permanganate is KMnO4.
Preparation Method
When the potassium hydroxide is along with the water is reacted with the manganese oxide. It gives the water as the byproduct. By electrolytic oxidation the manganete is converted into the permanganate. Because it is an alkaline metal. The acid can be induced in the disproportional reactions. Under the acidic conditions it is oxidized by the chlorine to get the permanganate and the potassium chloride is the byproduct.
Physical Properties
| Melting point | 240C |
| Boiling point | 100C |
| Molecular weight | 158.034g/mol |
| Density | 2.703g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.59 |
| Crystal structure | Orthorhombic |
| Magnetic susceptibility | +20.0×10-6cm3/mol |
| Appearance | Purplish bronze gray |
| Odor | Odourless |
| Solubility | Decomposes in alcohol and organic solvents |
Chemical Properties
Potassium permanganate is reaction with the acids and and contains the redox reactions. It can be only reduced by the three electrons. It is solubility in alcohol and organic solvents. It looks like a purplish bronze gray in the appearance. It is orthorhombic in nature. It has a low density and high molecular weight.
Uses
Potassium permanganate is used qualitative analysis to determine permanganate value. This is a compound used for disinfectant to cure skin diseases. Potassium permanganate is used in well water for treatment for removal of hydrogen sulphide and iron. KmnO4 is used for leather tanning and printing fabrics.