Potassium Iodate
Potassium iodate is an oxidizing agent which is the composition for potassium and iodate. It is a mixture of the ratio at 1:1. The potassium has the positively charged ions and iodate has the negatively charged ions. It is the primary source of dietary iodine. The systematic IUPAC name is known as potassium iodate. The chemical or molecular name of potassium iodate is KIO3.
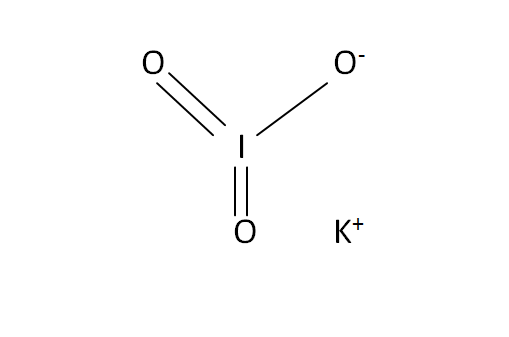
Structural Formula
This is the structural formula of the potassium iodate:
Chemical Formula
The chemical formula of the potassium iodate is KIO3. It has one potassium ions and three oxygen ions.
How the Potassium Iodate is Prepared
Potassium oxide is a basic agent. It can be prepared by the reaction between the potassium hydroxide with the iodic acid, it gives the formation of potassium iodate is the product and water as the byproduct. It crystallized at the point of combustible materials to caused the fire. The chemical reaction is given as follows.
HIO3 + KOH → KIO3 + H2O
Physical Properties
| Melting point | 560C |
| Boiling point | Decomposes |
| Molecular weight | 214.001g/mol |
| Density | 3.89g/cm3 |
| Solubility in water | 4.74g/100mL |
| Refractive index | 1.472 |
| Crystal structure | cubic |
| Magnetic susceptibility | -63.1×10-6cm3/mol |
| Appearance | White crystalline powder |
| Solubility | Soluble in KI solution. Insoluble in alcohol. |
| Odor | odorless |
Chemical Properties
It has a mousy odour and it is in the form of white crystalline powder. It is a non inflammable substances. It could be decomposed at the boiling point. The melting point is normal at the room temperatures. It is odourless. It has bitter in taste.
Applications
Potassium iodate is used for manufacturing the medicines. It is testing in arsenic and zinc. It is a reagent for feed additives. Potassium iodate is used for food as maturing agent and dough conditioner.