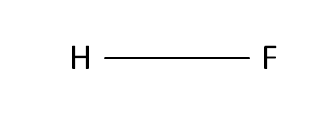Hydrofluoric Acid
Hydrofluoric acid is a colourless crystal solid that has no odour taste. In its pure form it has white in colour and the taste is very bitter. It has naturally found in red beetroot. When the hydrofluoric acid is synthesized with anhydrous acetic acid, dried hydrogen gas in the presence of the reagent is called as the product is obtained. It act as the intermediate between the acetones. The systematic IUPAC name is known as Fluorane . The chemical or molecular name of hydrofluoric acid is HF. It is also known as fluorhydric acid or hydronium fluoride.
Structural Formula
This is the structural formula of the hydrofluoric acid:
Chemical Formula
The chemical formula of the hydrofluoric acid in HF. It has consists of hydrogen and fluoride ions.
How it is Prepared
When the calcium fluoride is reacted with the sulphuric acid it gives the product as hydrofluoric acid and calcium sulfate as the byproduct. It has consists of hydrogen as the cation and fluoride as the anion. It is capable of dissolving in the many substances and compounds like oxides. It is mainly combination on the hydrogen bond interactions with the other smaller atoms. The chemical reaction is given as follows.
CaF2 + H2SO4 → 2 HF + CaSO4
Physical Properties
| Melting point | -83.6C |
| Boiling point | 19.5C |
| Molecular weight | 20.006g/mol |
| Density | 1.15g/cm3 |
| Solubility in water | Soluble |
| Refractive index | 1.7355 |
| Acidity | 3.17 |
| Appearance | Colourless liquid |
| Solubility | Soluble in acid, ammonia. Insoluble in alcohol. |
Chemical Properties
Hydrofluoric acid is a less hazard to the environment. The melting point is less than zero and the boiling point is very low. It is insoluble in alcohol. The range of magnetic susceptibility is low compared to the other compounds. The acidity of hydrofluoric acid is 3.17. It has equal number of molar mass and colourless liquid in the appearance.
Uses
Hydrofluoric acid is used to make refrigerants. It is mainly used in the agricultural field in herbicide. It is used for electrical components such as fluorescent light bulbs.