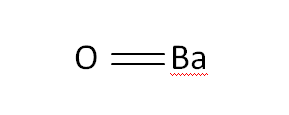Barium Oxide
Barium oxide is a non inflammable compound and it has been mostly used in the cathode ray tubes. It has contain highly excessive quantities that leads to the death. The barium has positively charged as the cation and oxide has negatively charged as the anion. It has the combination of barium and oxide. The systematic IUPAC name is known as barium oxide. The chemical or molecular name of barium oxide is BaO. The other names are barium protoxide, calcined baryta and baria.
Structural Formula
This is the structural formula of the barium oxide:
Chemical Formula
The chemical formula of the barium oxide is BaO. It has consists of one barium ions and one oxygen ions.
What Method the Acetamide Can Be Produced
Barium oxide can be prepared by the heating barium carbonate at the temperature between 1000 to 1450C. it gives the result of barium oxide is the product and carbon dioxide is the byproduct. In the another method, the barium nitrate is heated by thermal decomposition or barium carbonate is heated with the coke, carbon black or tar. The chemical reaction is given as follows.
2Ba + O2 → 2BaO
BaCO3 → BaO + CO2
Physical Properties
| Melting point | 1923C |
| Boiling point | -2000C |
| Molecular weight | 153.326g/mol |
| Density | 5.72g/cm3 |
| Solubility in water | 3.48g/100mL |
| Crystal structure | cubic |
| Magnetic susceptibility | -29.1×10-6cm3/mol |
| Appearance | White solid |
| Solubility | Soluble in ethanol, dilute mineral acids and alkalies. Insoluble in acetone and liquid ammonia. |
| Coordination geometry | octahedral |
Chemical Properties
Barium oxide is a non inflammable substances. It has high melting point and boiling point is less than zero that means it is negative. It has octahedral in coordination geometry. It is soluble in ethanol and insoluble in acetone. It looks like a white solid in the appearance.
Uses
Barium oxide is used to coat cathodes. Barium oxide is used to oxidize and also fuels. It is mainly used in the production of optical crown glass. For making the process of isomer separation.